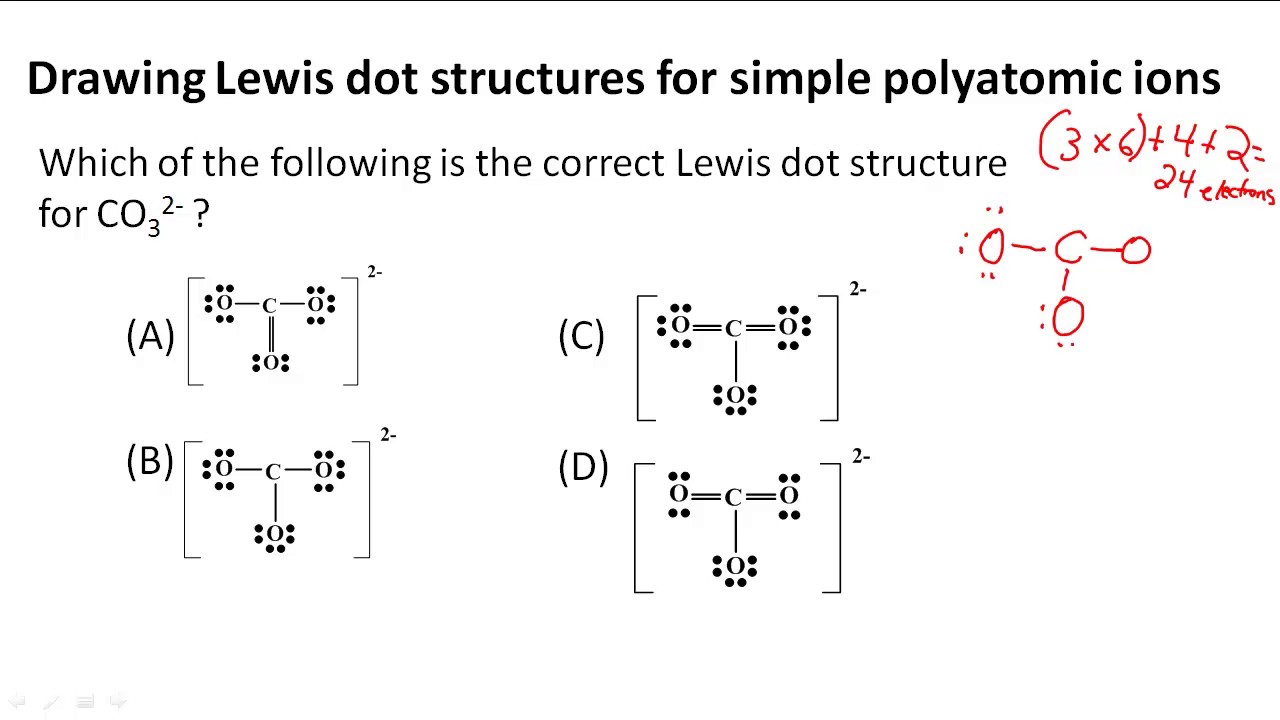
Understanding The Lewis Dot Structure Ion: A Comprehensive Guide
The Lewis dot structure ion is a fundamental concept in chemistry that helps in visualizing the arrangement of electrons around atoms and ions. This representation is an essential tool for understanding chemical bonding and the behavior of ions in different chemical reactions. By using dots to represent valence electrons, the Lewis dot structure simplifies the process of predicting how atoms will interact with one another, forming compounds and ions that are prevalent in nature.
The significance of the Lewis dot structure ion extends beyond mere representation; it provides insights into the stability and reactivity of ions. Whether you're a student delving into the world of chemistry or a seasoned expert revisiting the basics, grasping the concept of Lewis structures is crucial. This guide will explore the intricacies of Lewis dot structures, focusing specifically on ions, their formation, and their importance in chemical equations.
As we embark on this exploration of the Lewis dot structure ion, we will address common questions and misconceptions, provide illustrative examples, and discuss the relevance of these structures in real-world applications. Whether you're looking to understand how ions form or how to draw Lewis dot structures effectively, this article will serve as your comprehensive resource.
What are Lewis Dot Structures?
Lewis dot structures are symbolic representations of the valence electrons in an atom or ion. Named after the American chemist Gilbert N. Lewis, these structures use dots to denote the electrons surrounding the nucleus of the atom. This simplification allows chemists to easily visualize how atoms will bond with one another.
Why are Lewis Dot Structures Important for Ions?
The importance of Lewis dot structures for ions lies in their ability to depict the electron configuration that results from the loss or gain of electrons. Ions are charged particles that form when atoms either lose or gain electrons, resulting in a positive or negative charge. Understanding the Lewis dot structure ion helps elucidate how these charged particles interact with other atoms in chemical reactions.
How Do You Draw a Lewis Dot Structure for an Ion?
Drawing a Lewis dot structure for an ion involves several steps:
- Determine the total number of valence electrons available.
- Assign electrons to the central atom (if applicable) and surrounding atoms, following the octet rule.
- Account for any charges by adding or removing electrons to reflect the ion's charge.
- Draw the structure showing the arrangement of electrons around the atoms.
What is the Octet Rule in Relation to Ions?
The octet rule is a guiding principle in chemistry that states that atoms tend to bond in such a way that they each have eight electrons in their valence shell. This rule is particularly relevant for ions, as it helps explain why certain atoms gain or lose electrons. For example, sodium (Na) has one electron in its outer shell and tends to lose that electron to achieve a stable octet configuration, resulting in a positively charged ion (Na+).
What Are Some Common Examples of Lewis Dot Structures for Ions?
Several common ions can be illustrated using Lewis dot structures:
- Sodium Ion (Na+): Sodium loses one electron, resulting in a Lewis structure with no dots.
- Chloride Ion (Cl-): Chlorine gains one electron, resulting in a structure with eight dots representing its valence electrons.
- Calcium Ion (Ca2+): Calcium loses two electrons, represented by a structure with no dots.
- Oxide Ion (O2-): Oxygen gains two electrons, resulting in a structure with eight dots.
How Do Lewis Dot Structures Help in Predicting Chemical Reactions?
Lewis dot structures are invaluable in predicting the outcomes of chemical reactions. By visualizing the arrangement of electrons, chemists can anticipate how atoms will interact during bonding. This ability is particularly useful in organic chemistry, where reactions often involve the formation and breaking of covalent bonds.
Can Lewis Dot Structures Help in Understanding Molecular Geometry?
Absolutely! While Lewis dot structures primarily focus on electron configuration, they also provide insights into molecular geometry. The arrangement of electrons around an atom influences the shape of the molecule. For example, the presence of lone pairs of electrons can affect bond angles and the overall geometry of the molecule. Understanding these geometrical relationships is crucial for predicting the behavior of molecules in various chemical contexts.
What Resources Can Help in Learning About Lewis Dot Structures?
For those looking to deepen their understanding of Lewis dot structures, several resources are available:
- Textbooks: Many chemistry textbooks cover Lewis structures extensively, often with exercises and examples.
- Online Tutorials: Websites and video tutorials offer step-by-step guidance in drawing Lewis structures.
- Practice Worksheets: Printable worksheets provide problems to solve, reinforcing the concepts learned.
- Study Groups: Collaborating with peers can enhance understanding through discussion and shared insights.
In conclusion, the Lewis dot structure ion is a pivotal concept in chemistry that reveals the intricate relationship between electron configuration, bonding, and molecular behavior. By mastering this concept, students and professionals alike can unlock a deeper understanding of chemical interactions, paving the way for success in the field of chemistry.